
Process validation should be understood, beyond the traditional ‘three consecutive batches’, as the documented evidence of a solid scientific knowledge and understanding of the process, considering the critical parameters of the process and how these affect the quality attributes, the risks on the product and the process and the control mechanisms established for their prevention throughout their Lifecycle.
Products developed under a Quality by Design (QbD) approach, where the control strategy for the process can be scientifically and comprehensively defined, continuous manufacturing can be used and and then, continuous process verification can replace the traditional validation approach.

From the FDA’s ‘Risk-based approach’ initiative in 2002 to the present day and with the entry into force of new FDA guidelines (2011), EMA (2014) and the update of EU GMP Annex 15, there have been significant changes in the validation of manufacturing processes.
In fact, the “three-stages” approach, highlighting the incorporation of the ongoing process verification (EU) or continued process verification (FDA) stage and the relevance given to the statistical processing of process and product data, are dramatically changing methodologies and approaches to address this essential requirement of GMP.
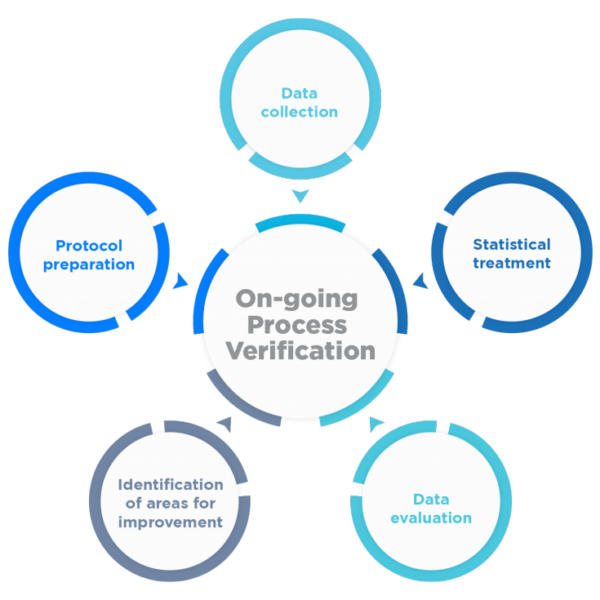
The main objective becomes to continuously ensure that the process remains in an adequate state of control during commercial batch manufacturing, as well as to facilitate the identification of necessary changes in the control strategy to drive continuous product and process improvement.
To this end, a program for the collection and analysis of process and product data and their continuous quality must be established.

Rephine has extensive experience at the international level in Process Validation, supported by numerous projects and successful inspections of our customers by the European health authorities and the FDA (Pre-Approval/Regular Inspections), including among others:
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.