- Global Audit Library
- Services
Our comprehensive support for full compliance of medical devices, offering you the peace of mind you deserve. + More
- About Us
- Resource Hub
- Login
- Contact Us
Our comprehensive support for full compliance of medical devices, offering you the peace of mind you deserve. + More
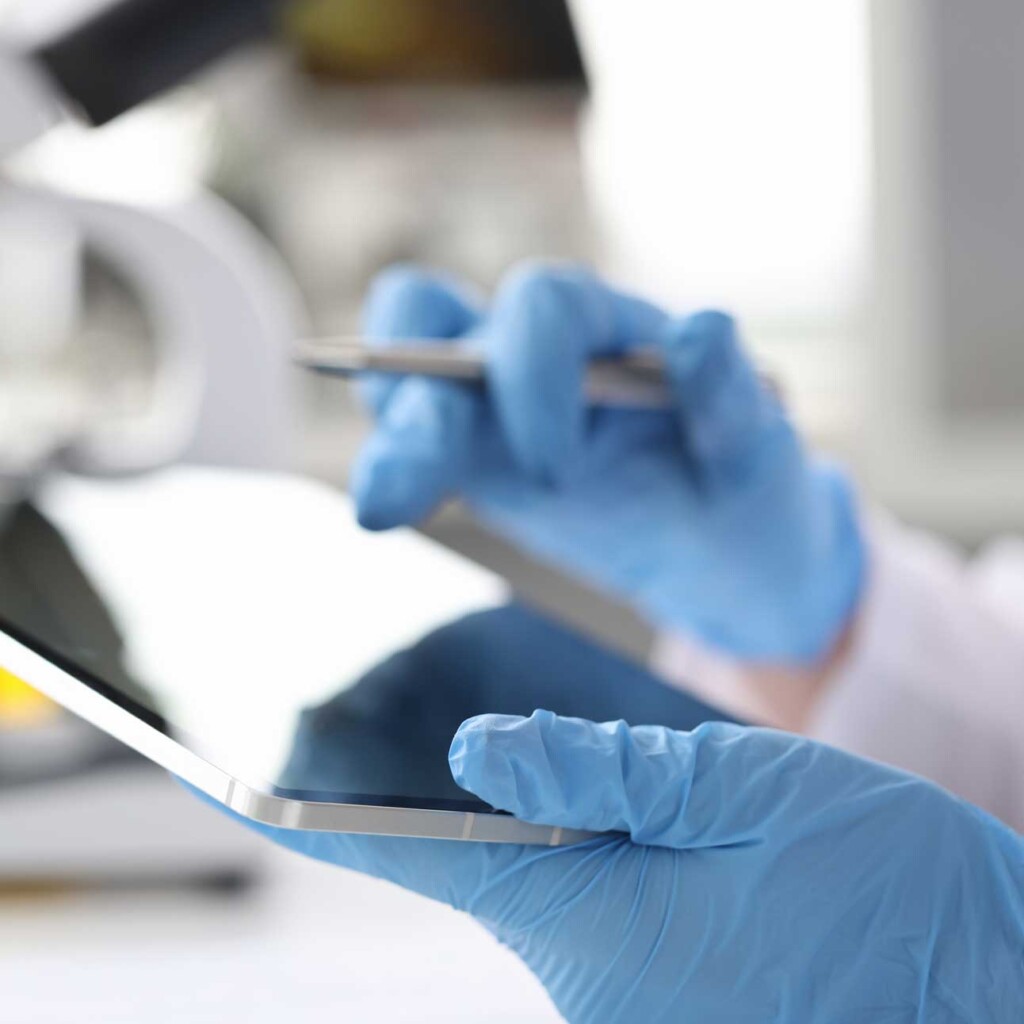
We have been auditing pharma manufacturers for more than 25 years. With our honed processes, attention to detail, and report consistency, we are widely acknowledged to be the Gold Standard in our field. Rephine performs various GMP audits including Active Pharmaceutical Ingredients (APIs) and Intermediates to ICH Q7 and ICH Q9 standards, as well as excipient, packaging and various other audits.
Comprehensive, high quality, customised, and fully independent reports.
Your information managed securely is an integral part of our process.
Reports accepted
by Regulatory, Health, and
Compliance Authorities.
Reports delivered and live
in days through the Rephine
Audit Library.
Carried out by specialised, highly experienced
and qualified auditors.
Designed specifically to cover whole sites; including QMS, facilities, and resources.
We offer the single largest repository of reports within three year validity, spanning more than 2,500 individual products, so whatever you’re looking for there’s a good chance we’ve already got it covered.
We conduct rigorous audits of pharma manufacturers across the supply chain, to ensure strict GMP standards are being fulfilled for applicable regulations and ensuring patient safety.


Our deeply-experienced Regulatory experts can provide help right
across the regulatory lifecycle, to ensure that dossiers are ready for market delivery and maintain ongoing compliance submission.
Reduce your audit burden with our comprehensive Auditee support service, we aim to free up Auditee internal resource for efficient and effective audits all whilst reducing costs and streamlined CAPA management.

We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.