
Our proven methodology ensures that audits are conducted efficiently and effectively, leading to a reduction in both resources and costs associated with hosting audits.
Rephine’s streamlined process reduces the number of audits to respond to, freeing up your internal resource to focus on other quality related matters. Our dedicated team of Technical Managers will work with you from audit preparation to closure.
This member of the Rephine Library has benefited from a huge reduction in their audit burden and saved significant resource in time and money through Rephine’s proven audit methodology.
The Group has trusted Rephine’s dedication to confidentiality, impartiality and high-quality standards demonstrated through our excellent team of auditors.
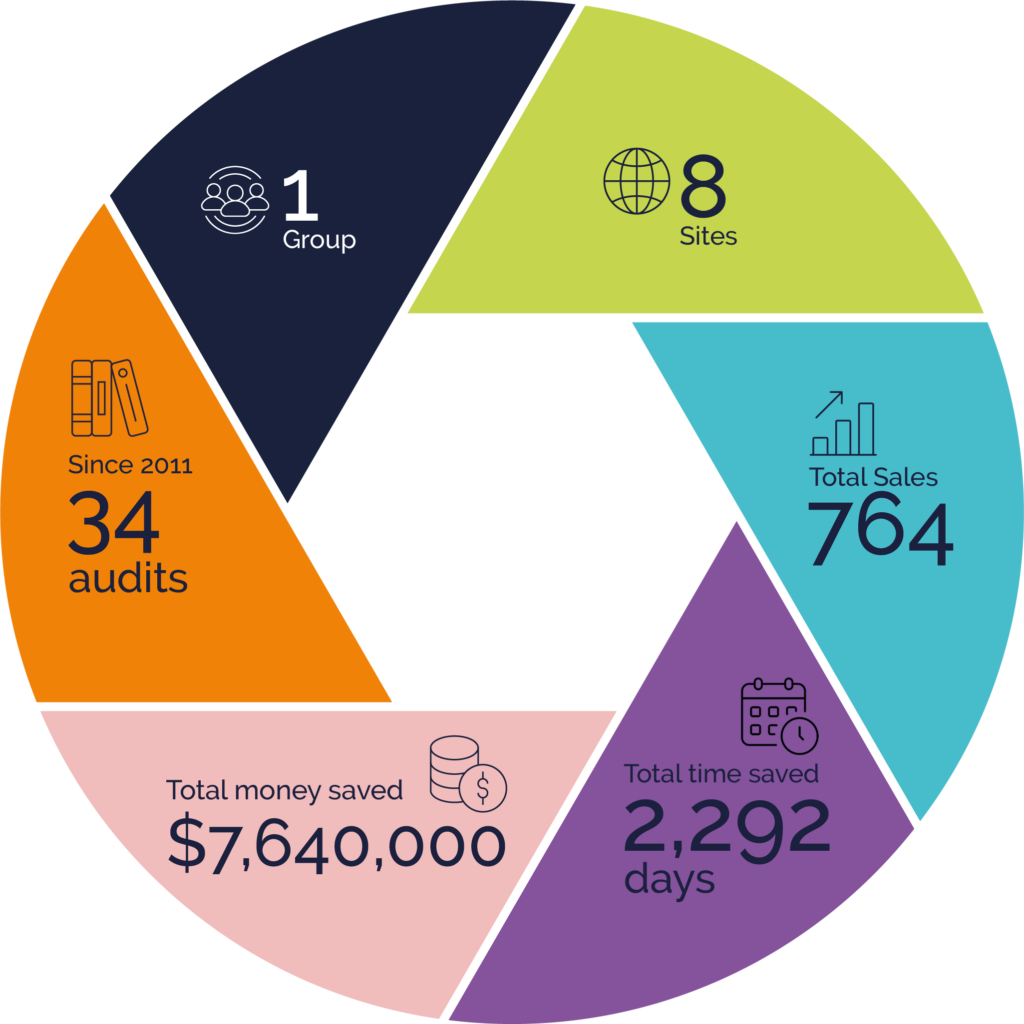
Total audits conducted by
Rephine since 2011, re-
audited on a 3 year cycle.
Parent company
API manufacturing
facilities across the world
Reports shared with different
clients across all sites/audits
An estimate of number
of days saved at the auditee site
dedicated to hosting audit based
on 3 days/audit
An estimate of the money saved
at the auditee site dedicated to
hosting audit based on
$10,000/audit
Source: Rephine Survey
We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.