- Global Audit Library
- Services
Our comprehensive support for full compliance of medical devices, offering you the peace of mind you deserve. + More
- About Us
- Resource Hub
- Login
- Contact Us
Our comprehensive support for full compliance of medical devices, offering you the peace of mind you deserve. + More
The methodology for commissioning and qualification is based on coordinated work with equipment and service suppliers, the engineering in charge and the engineering department of the property.

This means good operational procedures, methods, instructions, trained personnel and a good qualification of the equipment, facilities and services involved in the process.
Qualification, carried out according to a methodology of risk management (ICH Q9) in order to focus on the critical aspects of each qualified element and, at the same time, to optimize time and resources, constitutes a key factor to provide the necessary evidence of knowledge and control of the equipment and of the process environment
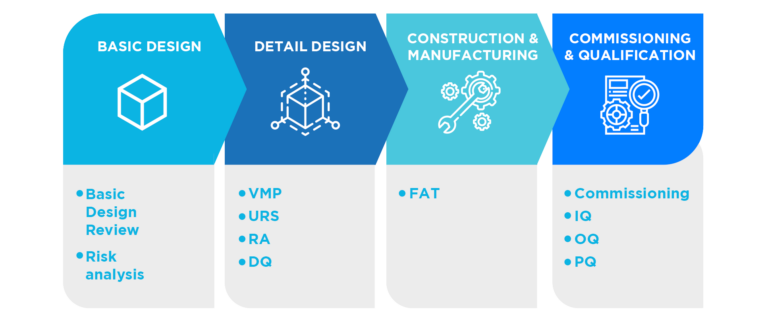
In this way, we provide the client with the necessary experience and knowledge to meet their GMP compliance objectives in the design, commissioning and qualification stages. In addition we integrate quality in the project with validation activities, standardize quality standards and qualification documentation and optimize the resources of the Engineering departments.

We provide extensive GMP consulting services to help keep our clients ahead of the needs and expectations of regulators.
Explore our extensive GMP audit library to see the range and scope of live reports we have in stock, join a live audit, or commission a bespoke audit
Maintain high standards of life sciences manufacturing supplier qualifications and GMP auditing within the supply chain through our expertise
Discover how we can help your product reach to market, fully and demonstrably complying with the latest GxP standards
From data integrity to implementing new systems, our experienced team with a digital mindset, can lead you to transformative achievements
REPHINE CHINA
REPHINE INDIA
Sign up to our newsletter to get the latest news about Rephine and industry news.